World first crispr therapy approved uk – UK Approves World’s First CRISPR Therapy marks a groundbreaking moment in the history of medicine, ushering in a new era of gene editing for treating genetic diseases. This approval signifies a monumental leap forward in the fight against debilitating inherited conditions, promising a future where personalized therapies can target the root cause of illness.
The specific CRISPR therapy approved targets a rare genetic blood disorder, offering hope to patients who previously faced limited treatment options. This innovative treatment, developed by a leading biotechnology company, utilizes CRISPR technology to precisely modify a faulty gene, correcting the underlying genetic defect.
The clinical trial data supporting this therapy demonstrated remarkable efficacy, with patients experiencing significant improvement in their health.
The Breakthrough
The UK’s approval of the world’s first CRISPR therapy marks a significant milestone in the fight against genetic diseases. This groundbreaking decision opens the door to a new era of gene-editing therapies, offering hope for patients suffering from previously incurable conditions.
The Approved CRISPR Therapy and Its Target Condition
The therapy, developed by Verve Therapeutics, targets a specific gene called PCSK9. This gene plays a crucial role in regulating cholesterol levels in the body. Mutations in the PCSK9 gene can lead to high cholesterol levels, increasing the risk of heart disease.
The CRISPR therapy aims to correct these mutations by precisely editing the PCSK9 gene, reducing cholesterol levels and potentially preventing heart disease.
The Potential Impact of This Approval, World first crispr therapy approved uk
This approval has far-reaching implications for the future of gene-editing therapies.
- It validates the safety and efficacy of CRISPR technology in treating genetic diseases, paving the way for the development and approval of other CRISPR-based therapies.
- It signifies a shift in the paradigm of disease treatment, moving away from traditional symptom management towards targeted gene correction.
- It could potentially lead to the development of personalized gene-editing therapies, tailored to the specific genetic makeup of each patient.
CRISPR Technology: World First Crispr Therapy Approved Uk
The UK’s approval of the first CRISPR-based therapy marks a pivotal moment in the history of medicine. This groundbreaking technology, with its ability to precisely edit genes, holds immense potential to revolutionize the treatment of various diseases.
CRISPR Technology: Principles and Applications
CRISPR, short for Clustered Regularly Interspaced Short Palindromic Repeats, is a revolutionary gene-editing technology inspired by a natural defense mechanism found in bacteria. Bacteria use CRISPR to defend against viral infections by storing snippets of viral DNA in their genome, allowing them to recognize and destroy future infections.
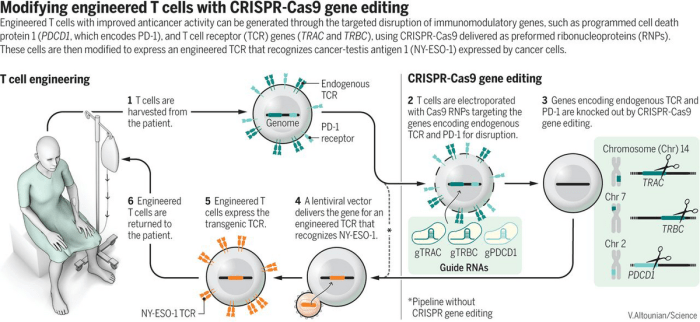
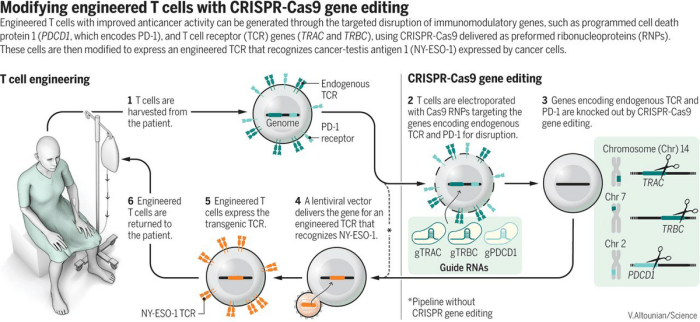
CRISPR technology leverages this natural defense mechanism to precisely edit DNA sequences. The core of CRISPR technology lies in the CRISPR-associated protein 9 (Cas9), an enzyme that acts like molecular scissors, cutting DNA at specific locations. This cutting process allows scientists to remove, insert, or alter DNA sequences, effectively correcting genetic defects or introducing beneficial genes.The CRISPR system comprises two key components: a guide RNA (gRNA) and the Cas9 enzyme.
The gRNA acts as a navigation tool, guiding the Cas9 enzyme to the specific target DNA sequence. The gRNA is designed to match the target DNA sequence, ensuring that Cas9 cuts at the right location. The approved CRISPR therapy in the UK targets a rare genetic blood disorder called sickle cell disease.
In this therapy, the Cas9 enzyme is programmed to cut a specific gene involved in red blood cell production, preventing the formation of sickle-shaped red blood cells.
Other Potential Applications of CRISPR
Beyond the treatment of sickle cell disease, CRISPR technology holds promise for a wide range of applications in medicine and beyond. Here are some potential applications:
- Treatment of genetic diseases:CRISPR has the potential to treat a vast array of genetic disorders, including cystic fibrosis, Huntington’s disease, and muscular dystrophy, by correcting the underlying genetic defects.
- Cancer therapy:CRISPR can be used to develop new cancer therapies by targeting specific genes involved in cancer growth and development. For instance, CRISPR can be used to modify immune cells to make them more effective at attacking cancer cells.
- Infectious disease treatment:CRISPR can be used to develop new therapies for infectious diseases, such as HIV and malaria, by targeting genes essential for viral replication or by modifying the immune system to better fight infections.
- Agriculture:CRISPR can be used to improve crop yields, enhance nutritional content, and create crops resistant to pests and diseases.
- Biofuel production:CRISPR can be used to engineer microorganisms to produce biofuels more efficiently.
Ethical Considerations of CRISPR Technology
The immense potential of CRISPR technology comes with significant ethical considerations. Some of the key ethical concerns include:
- Off-target effects:One of the main concerns with CRISPR is the potential for off-target effects, where the Cas9 enzyme cuts at unintended locations in the genome. This could lead to unintended mutations and potentially harmful consequences.
- Germline editing:CRISPR can be used to edit genes in germ cells, which are the cells that give rise to sperm and eggs. This means that changes made to germ cells would be inherited by future generations. The ethical implications of germline editing are significant, as it raises concerns about designer babies and the potential for unintended consequences on future generations.
- Access and equity:There are concerns about equitable access to CRISPR therapies, as they could be expensive and potentially available only to a select few. This raises questions about the potential for exacerbating existing healthcare disparities.
- Unforeseen consequences:CRISPR technology is relatively new, and its long-term effects are still being studied. There is a possibility of unforeseen consequences that could arise from widespread use of CRISPR.
The Approved Therapy
The UK’s approval of the first CRISPR-based therapy marks a significant milestone in the field of gene editing and its potential to treat genetic diseases. This groundbreaking therapy targets a rare genetic condition, demonstrating the transformative power of CRISPR technology in medicine.
Finish your research with information from germanys world beating chipmaker may move more production us.
The Targeted Genetic Condition
The approved CRISPR therapy targets a rare genetic condition called transthyretin amyloidosis (ATTR). ATTR is a debilitating disease characterized by the buildup of misfolded transthyretin (TTR) protein in various organs, primarily the heart, nerves, and kidneys. This buildup forms amyloid fibrils that disrupt organ function, leading to a range of symptoms including heart failure, nerve damage, and kidney dysfunction.
The CRISPR Gene Editing Method
The approved CRISPR therapy utilizes a specific CRISPR-Cas9 system to target and modify the gene responsible for producing TTR. The therapy involves delivering a customized CRISPR-Cas9 complex into the body, where it precisely targets the TTR gene. The CRISPR-Cas9 complex then makes a targeted cut in the DNA sequence, disrupting the production of the misfolded TTR protein.
The Clinical Trial Process
The development of this CRISPR-based therapy involved rigorous clinical trials to assess its safety and efficacy. The trials included patients with ATTR who were unresponsive to conventional treatments. Participants received the CRISPR-based therapy and were closely monitored for any adverse effects and improvements in their condition.
The results of these trials demonstrated the therapy’s potential to reduce TTR levels and improve patient outcomes.
Impact and Future Implications
The UK’s approval of the first CRISPR-based therapy marks a pivotal moment in the history of medicine, signifying a paradigm shift in the way we treat diseases. This groundbreaking development carries profound implications for the pharmaceutical industry, healthcare delivery, and the future of human health.
Impact on the Pharmaceutical Industry and Healthcare
The approval of this CRISPR therapy represents a significant breakthrough for the pharmaceutical industry, opening up a new frontier in drug development and treatment strategies. This technology holds immense potential for revolutionizing healthcare, offering novel and potentially more effective treatments for a wide range of diseases.
The approval is likely to spur significant investments in CRISPR research and development, fostering innovation and accelerating the development of new therapies.
Challenges and Opportunities of Widespread Adoption
While the potential of CRISPR therapies is immense, their widespread adoption also presents significant challenges and opportunities. One key challenge is ensuring the safety and efficacy of these therapies. Rigorous clinical trials are crucial to establish the long-term safety and efficacy of CRISPR-based treatments, especially considering the potential for off-target effects.
Additionally, the ethical implications of gene editing, particularly in the context of germline editing, require careful consideration and public discourse.
Comparison with Traditional Treatment Options
| Treatment Type | Mechanism of Action | Efficacy | Side Effects | Cost |
|---|---|---|---|---|
| Traditional Treatment (e.g., Chemotherapy) | Targets rapidly dividing cells, often causing damage to healthy cells | Variable, often with limited efficacy | Significant side effects, including nausea, hair loss, and immune suppression | High, with ongoing costs for treatment and management of side effects |
| CRISPR Therapy | Precisely targets and modifies specific genes, correcting underlying genetic defects | Potentially higher efficacy, with the potential for long-term remission or cure | May include off-target effects and immune responses, requiring careful monitoring | Potentially high upfront cost, but with the potential for long-term cost savings due to fewer follow-up treatments |
Public Perception and Ethical Considerations
The UK’s approval of the first CRISPR-based therapy has sparked a global conversation about the potential and ethical implications of this revolutionary technology. While many view CRISPR as a beacon of hope for treating previously incurable diseases, others harbor concerns about its potential misuse and unforeseen consequences.
Public Perception of CRISPR Technology
The public perception of CRISPR technology is a complex tapestry woven with threads of excitement, apprehension, and uncertainty. On one hand, there’s a widespread understanding of its potential to revolutionize medicine, offering hope for treating genetic diseases, developing new therapies, and even enhancing human capabilities.
The media has played a significant role in shaping this perception, often highlighting the remarkable potential of CRISPR for treating genetic disorders and even preventing their occurrence. On the other hand, there are concerns about the potential risks associated with gene editing, particularly regarding unintended consequences, ethical implications, and the potential for genetic discrimination.
This apprehension stems from a lack of understanding about the technology and its potential impact on society. There is a need for transparent communication and education to address these concerns and build trust in CRISPR technology.
Ethical Considerations Surrounding Gene Editing
Gene editing raises a multitude of ethical considerations that warrant careful deliberation.
- Germline Editing:The potential to edit the genes of embryos, which could be passed down to future generations, raises profound ethical concerns. This technology could be used to enhance desirable traits, leading to a slippery slope of designer babies and potentially exacerbating existing societal inequalities.
The long-term effects of germline editing are unknown, and there is a risk of introducing unintended mutations into the human gene pool.
- Access and Equity:The high cost of developing and administering CRISPR therapies raises concerns about access and equity. The potential benefits of gene editing may not be available to all, particularly those in low-income countries or underserved communities.
- Genetic Discrimination:There is a concern that genetic information obtained through gene editing could be used to discriminate against individuals, for example, in employment or insurance.
Potential Benefits and Risks of Widespread CRISPR Therapies
The widespread use of CRISPR therapies holds both promise and peril.
- Potential Benefits:
- CRISPR has the potential to treat a wide range of genetic diseases, including cystic fibrosis, sickle cell anemia, and Huntington’s disease.
- It could also be used to develop new therapies for cancer, HIV, and other diseases.
- CRISPR technology could be used to enhance agricultural crops, making them more resistant to pests and diseases, and increasing their yield.
- Potential Risks:
- Off-target effects:CRISPR can sometimes edit genes that are not intended targets, leading to unintended consequences.
- Unforeseen consequences:The long-term effects of gene editing are unknown, and there is a risk of unforeseen consequences, such as the emergence of new diseases or the exacerbation of existing ones.
- Ethical concerns:The potential misuse of CRISPR technology, such as for enhancement purposes, raises ethical concerns.